Ryosuke Okuno, Center for Subsurface Energy and the Environment, The University of Texas at Austin
The energy transition requires managing the global carbon balance with a large throughput of CO2 while shifting to less carbon intensive energy sources, such as hydrogen, in economically feasible, publicly acceptable ways. Many issues with processing CO2 and H2 come from their physical properties, their compression, transportation, and storage [e.g., H2 embrittlement]. Also, geological CO2 sequestration has various issues associated with the properties of CO2, such as low carbon density at low to moderate pressure, low mass density, low viscosity, immiscibility with water, and corrosivity [e.g., low pH near a CO2 plume]. In particular, CO2 injection often results in inefficient use of pore space in the formation under geophysical heterogeneities.
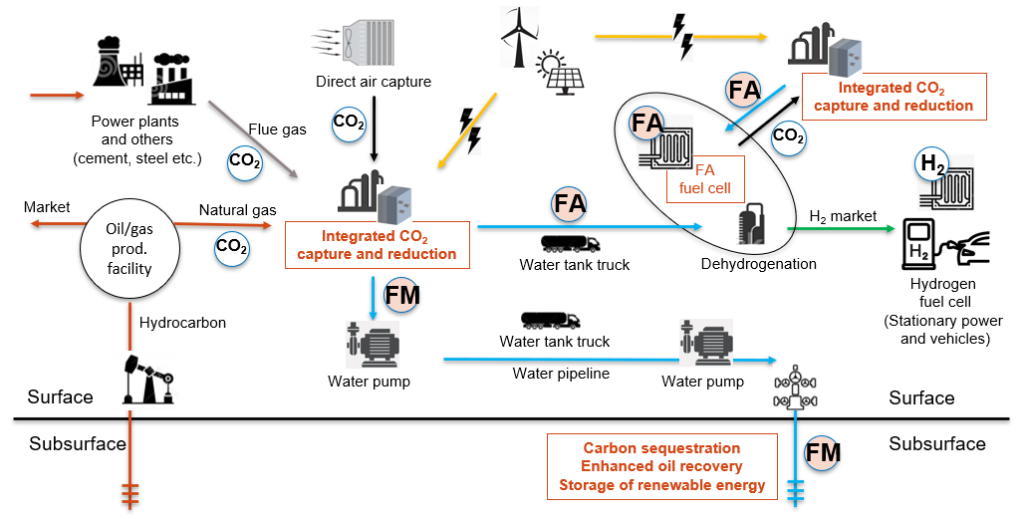
We have studied formate (FM or HCOO-) as a novel carbon carrier for improved carbon management and as a hydrogen carrier in the energy transition (Fig. 1). Use of aqueous FM solution can improve many issues with handling CO2 and H2, and enhance the efficiency of pore-space utilization in geological carbon sequestration without relying on structural- and capillary-trapping mechanisms. FM has been widely used in oil fields as a densifier for drilling fluids because of its desirable HSE profile, and can be generated via electrochemical reduction of CO2, the TRL of which is currently rated as six. FM can play critical roles in the energy transition as a carbon carrier in CCUS and as a hydrogen carrier for surface and subsurface applications.
- Carbon carrier
- EOR agent
- Hydrogen carrier
- Raw material for carbon products
- Hydrogen carrier
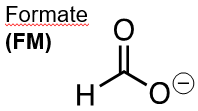
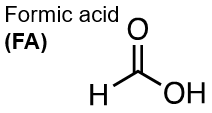
Fig. 1. Left: Formate ion (FM), Right: Formic acid (FA). The dominant species is FM at typical formation pH values (6 – 8), and FA at low pH (2 – 3). FM has been used in drilling fluids in oil fields and as an environmentally benign antifreeze in many regions.
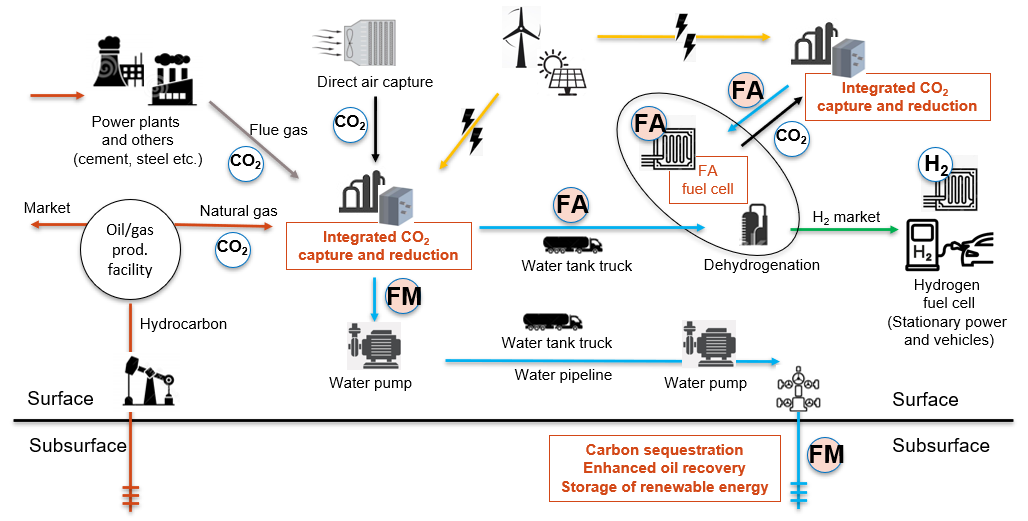
Figure 2. Potential scenarios of CCUS and hydrogen energy systems using FM and FA.
Key points about surface and subsurface applications of FM/FA
- One barrel of water (brine) can contain up to 126 kg of CO2-e carbon even at atmospheric pressure.
- FM is stable even at high-salinity, high-temperature (> 200°C) with/without rock minerals.
- Aqueous FM solution flows like ordinary water (Newtonian fluid).
- FM species slightly increase the solution viscosity (< 3 cp) at typical formation temperatures.
- Novel mechanisms have been found for wettability alteration of carbonate rocks with aqueous FM solution.
- FM/FA can be used to store renewable energy as an aqueous hydrogen carrier.