Energi Simulation IAP on Carbon Utilization and Storage, Ryosuke Okuno
Overview
This project aims to develop technologies of aqueous nanobubble (NB) dispersion of CO2 for geological carbon sequestration, primarily for CO2 mineralization in (ultra)mafic rock formations. We aim to substantially reduce the amount of water required to sequester a given amount of CO2 (e.g., by a factor of 2), leading to a reduced cost (capital and operational) of CO2 mineralization. Aqueous nanobubble dispersion also enables supersaturation of brine by CO2 beyond the inherent solubility and enhances the kinetics and extent of mineral dissolution and CO2 mineralization. In addition to fundamental studies of aqueous NB dispersion, we are developing suitable methods of aqueous CO2 NB generation for continuous operation at elevated pressure for CO2 mineralization. Primary collaborators on this topic include “44.01” and “Japan Petroleum Exploration”.
Background
Carbonated water has been used as a variant of waterflooding for oil recovery. It recently attracted attention for CO2 sequestration because carbonated water may accelerate the eventual mineralization of CO2 without needing the CO2 dissolution step, for example, in (ultra)mafic formations (e.g., basalt). An obvious shortcoming of using carbonated water is that the carbon density in carbonated water (i.e., CO2 solubility) is much smaller than pure CO2 at injection conditions (i.e., supercritical CO2), and the CO2 solubility decreases with salinity and temperature. An estimation based on CarbFix’s CO2 mineralization project is that 22 tonnes of water was required to capture 1 tonne of pure CO2 for storage; that is, the carbon density was approximately 1 mol/L, a common level of CO2 solubility in water.
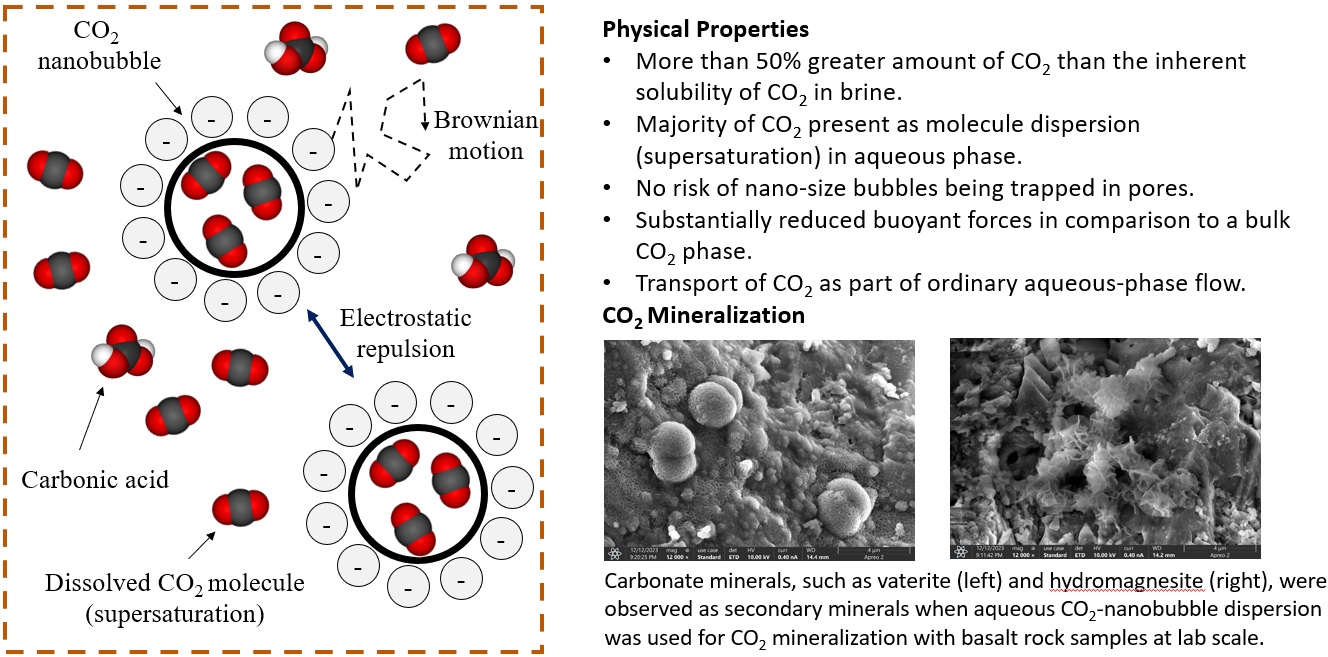
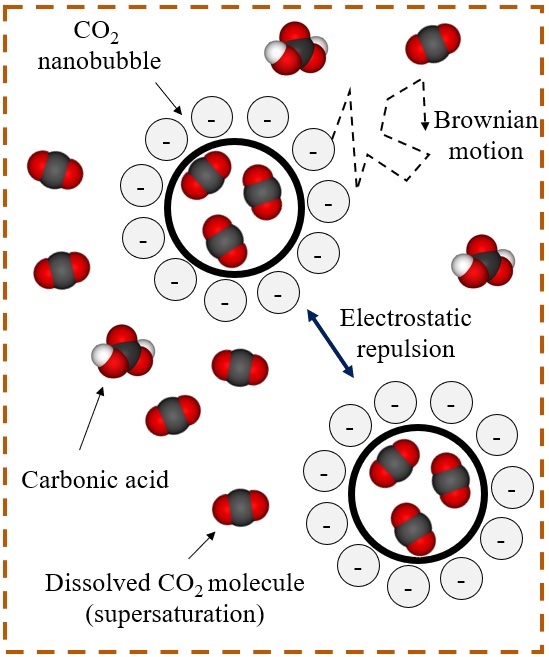
It is possible to increase the CO2 content in water beyond carbonated water by generating nanobubbles of CO2 dispersed in water. Aqueous nanobubble dispersion refers to a state in which nano-scale bubbles of water-immiscible gaseous species are dispersed in the external water phase supersaturated by the gaseous species. Nanobubble dispersions have been applied in various industries, such as agriculture and wastewater treatment, with the ability to contain a large amount of gas. Kinetically stable nanobubbles at atmospheric pressure in an open system have been reported because of extremely small buoyant forces, repulsive forces among negatively charged surfaces of nanobubbles, and the supersaturated water suppressing Ostwald ripening. However, the previous studies on nanobubble dispersions are focused on an open system near atmospheric pressure. We have uniquely studied the fundamentals of nanobubble dispersions for subsurface applications.